The TULSA-PRO Value

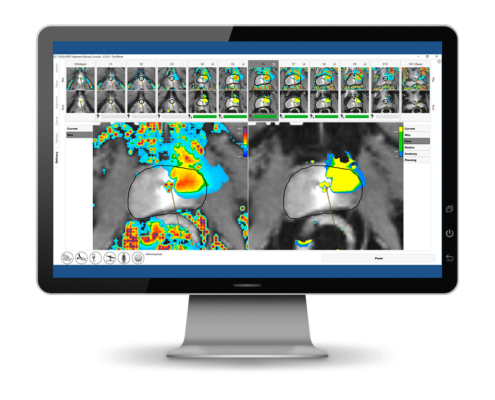
Real-time MR imaging
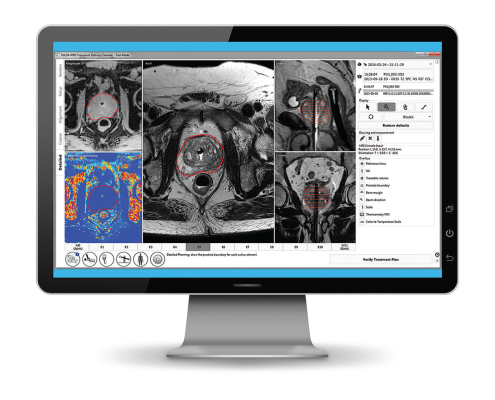
Customized treatment plan
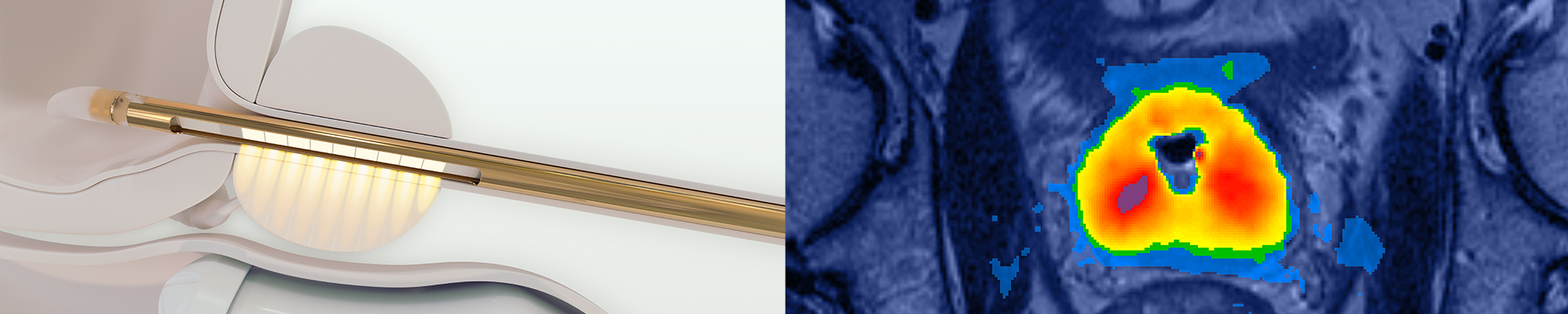
Transurethral directional ultrasound for thermal ablation; water cooling of urethra and rectum
Sweeping ultrasound, continuous rotation
Capable of treating both large and small prostate volumes, anterior and posterior prostate tissue
Thermal protection of important anatomy
Closed-loop process control software
Real-time temperature feedback provides for gentle and precise ablation
The TULSA-PRO® system is designed to provide customizable and predictable ablation of a surgeon defined region of prostate while actively protecting the urethra and rectum to help preserve the patient’s natural functional abilities.
Customizable
The flexibility to treat different types of patients
Treat a variety of patients in one day
The flexibility to treat each patient differently
‘My life should not have to change’
Each of your patients has unique needs, TULSA-PRO allows you to customize so your patient’s life does not have to change
Treat almost any size prostate
TULSA-PRO has been used to treat prostates up to 250cc
Predictable
Actively protect the urethra and rectum during treatment
Preserve natural functional abilities
The physician defines the treatment plan, the robot follows the physician
Preserve options for future treatments
Predictable prostate volume reduction
Ablate the disease
Incision-free
Real-time MRI guidance and closed-loop thermal feedback
Consistent procedure lengths
Consistently treat 4 patients a day
- High throughput
- Patient tolerability
- Single outpatient procedure – minimal pain, fast recovery
Cost
- MR Suite significantly less expensive to operate than an operating room
Directional Transurethral Ultrasound
Radiation free treatment
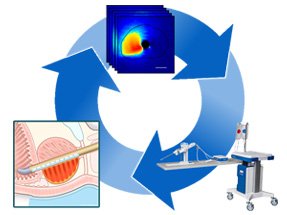
The TULSA-PRO Workflow
TULSA-PRO REIMBURSEMENT
For reimbursement guidance and/or access to our FREE reimbursement coding guide, please view and click the button below.
106732B
CAUTION: TULSA-PRO® is approved for commercial sale in the USA (K191200) & EU (CE 2797). Rx only device.
For all other Regulatory jurisdictions, please contact us at [email protected] for more information regarding the regulatory status of the device.