Single center retrospective analysis of fifty-two prostate cancer patients with customized MR-guided transurethral ultrasound ablation (TULSA)
Agron Lumiani M.D, Diyala Samun Dip.-Ing., Ronald Sroka Ph.D., Rolf Muschter M.D.
The TULSA Procedure is offered in routine clinical practice at the ALTA Klinik (Bielefeld, Germany) to men with prostate cancer seeking minimally invasive treatment. Results from a clinical service evaluation performed at the clinic, intended to assess benefit in the real-world setting, were published in Urologic Oncology. The report included 52 men with D’ Amico low (13%), medium (67%), or high-risk (19%) prostate cancer receiving the TULSA Procedure either as initial treatment for prostate cancer, or for recurrent disease after other modalities failed (2 HIFU, 1 laser+HIFU, 1 EBRT, 1 hyperthermia).
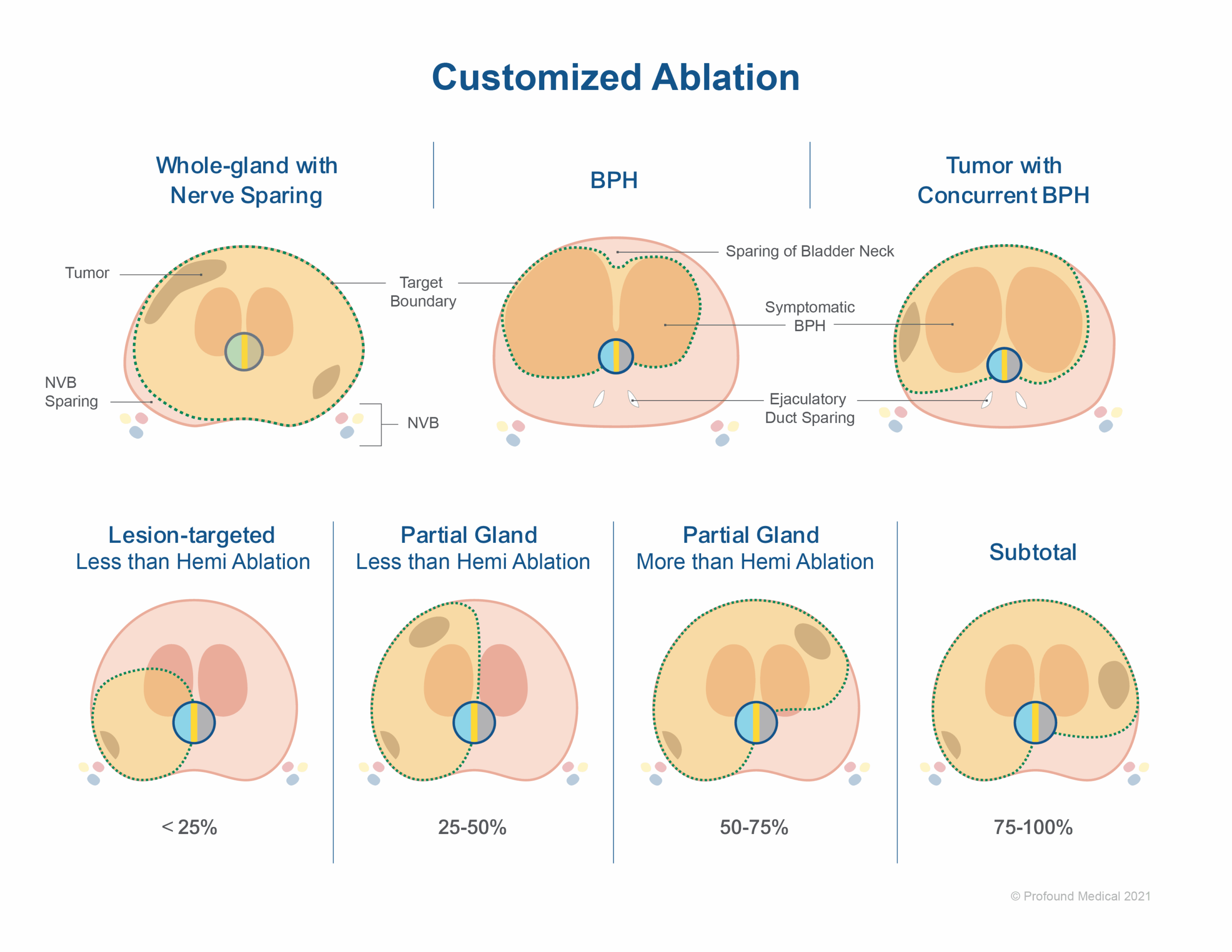
A combination treatment was delivered in 24/52 of the men (46%) targeting both cancer and benign prostatic hyperplasia (BPH) in a single procedure. Treatment plans were customized for patient-specific disease characteristics and to spare important anatomy. A 3 mm margin around the urinary sphincter was incorporated to reduce the risk of incontinence, and, where feasible, neurovascular bundles were spared to reduce the risk of impotence. According to the authors, the range of prostate volume at baseline was 17-122 cc and the fraction of gland targeted ranged from less than 50% to whole-gland (Figure 1). The following outcomes were assessed at median (IQR) 16 (12-22) months: early treatment success for prostate cancer, based on mpMRI, biochemical recurrence (Phoenix), and confirmatory biopsy; relief of LUTS (lower urinary tract symptoms, for men receiving combination treatment); adverse events; preservation of continence and potency.
After a single TULSA Procedure, early treatment success for prostate cancer was achieved in 38 men (73%). Of the nine men who elected for a repeat TULSA Procedure, 8 achieved early treatment success at follow up (median time=14 months) increasing the rate of early treatment success after first or second TULSA Procedure to 88%. The majority of men underwent partial ablation (80%). All men who were previously fully potent, and 44/45 (98%) of men with full or partial potency at baseline, maintained erectile function. Pad-free continence was preserved in 51/52 (98%) of men. After the combination treatment targeting the prostate cancer and BPH, improvement in LUTS was achieved for 20/24 (83%) of men. Two complications requiring intervention (without general anesthesia) were observed, and there were no bowel-related complications.
The authors note that partial ablation had better preservation of urinary continence and erectile function compared with whole-gland, and fewer adverse events. Within the subset of patients who incurred a recurrence after the first TULSA procedure, one man underwent a non-complicated radical prostatectomy. Patients with high-risk disease at baseline had higher risk of recurrence, and there was no recurrence among men with low-risk disease at baseline. The following causes for failure were identified: suboptimal screening for calcifications, and insufficient thermal margins.
Based on these results, the authors of this study found the TULSA Procedure to be safe and effective at treating low to high-risk prostate cancer in the real-world setting, and also at simultaneously relieving LUTS through customized treatment plans, both as a primary treatment or a salvage treatment delivered after other modalities fail. Repeat TULSA Procedure is feasible if needed to achieve definitive treatment.
To read more about this study, visit: https://www.sciencedirect.com/science/article/pii/S1078143921001733