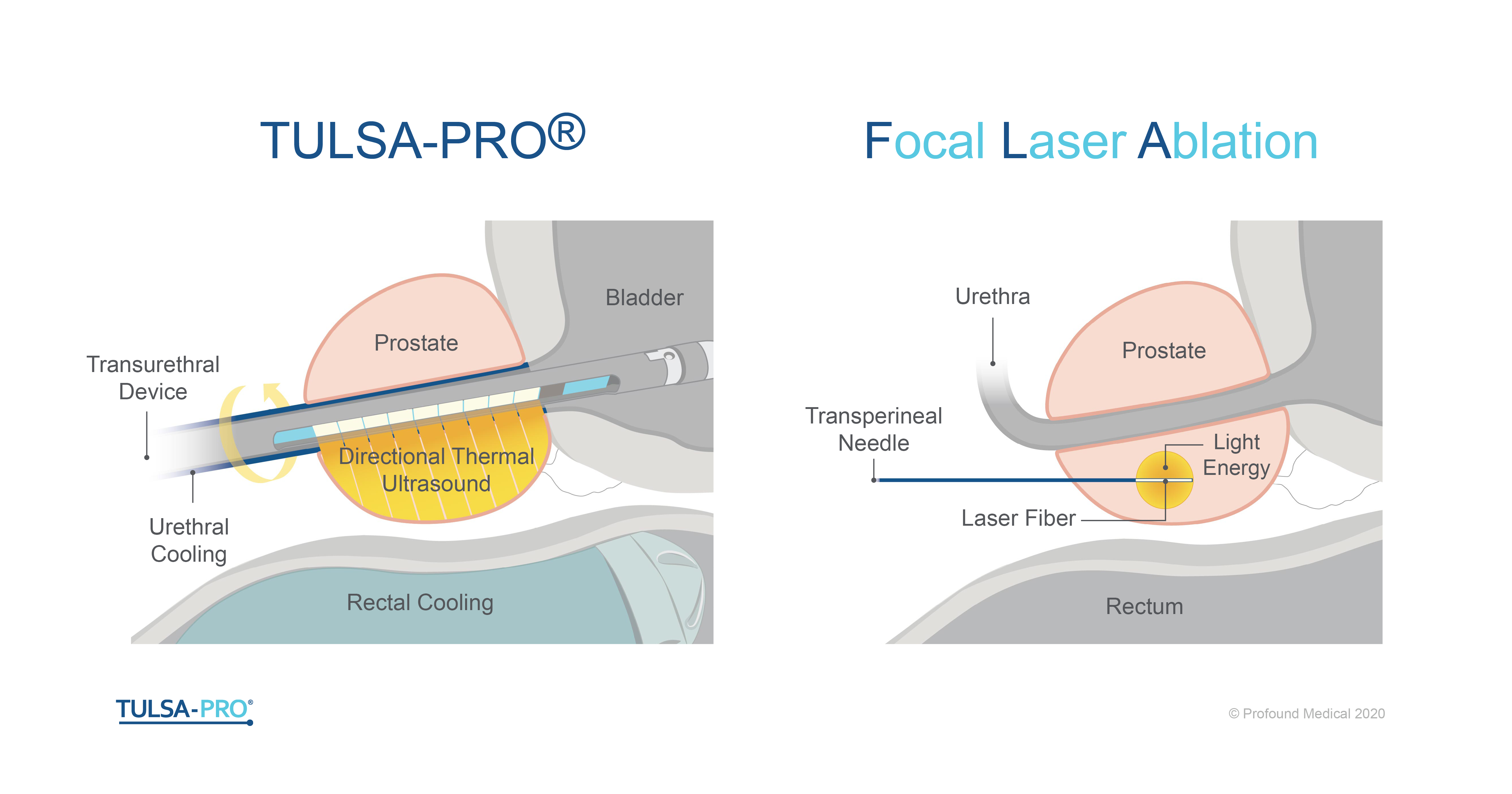
What is the difference between TULSA-PRO® and FLA?
FLA stands for focal laser ablation. It is also referred to as laser interstitial thermotherapy. FLA is a thermo-ablation technique where high-energy light is emitted from a laser to ablate targeted tissue by means of coagulative necrosis1. During FLA prostate ablation, this energy is delivered to the prostate using a laser fiber that is pushed into the prostate by a transperineal or transrectal approach along a needle guide. The very tip of the laser fiber emits light energy in a spherical area that gets absorbed by the prostate tissue, heating it up to high enough temperatures to ablate the prostate tissue2. This procedure is typically performed under the guidance of real-time MR imaging1. In one study, all men underwent MRI guided FLA targeting 1 or 2 lesions having tested positive for prostate cancer. With the estimated volume of each lesion being less than 2cm3, the maximum FLA ablation diameter was 15 millimeters (3). Larger zones are ablated by retracing the laser and repeating the ablation, or by inserting the laser in another location. In another study, two different sized laser applicators were used to ablate small and large tumors: the small applicator had a 1cm heat-diffusing tip, and the other had a 1.5cm heat-diffusing tip4. Another study with 24 months of follow-up data published in 2019 with the largest cohort for MRI guided FLA, has similar inclusion criteria regarding lesion size and volume5.
TULSA-PRO stands for Transurethral Ultrasound Ablation of the prostate. During the TULSA Procedure, thermal ultrasound energy is delivered directionally from a device within the urethra, known as the ultrasound applicator. The ultrasound heating extends to a radial depth of 30mm away from each of the ten independent 5mm-long elements, enabling ablation of regions with up to 60mm diameter and 50mm length. The device rotates and delivers the ultrasound heat in a sweeping pattern contacting a large volume of tissue in minimal time. The prostate tissue is therefore ablated from an ‘inside-out’ approach by delivering the directional, thermal ultrasound from the urethra outwards to the edge of the target region of prostate, while actively protecting the urethra and rectum through cooling mechanisms. The TULSA-PRO system combines directional, transurethral thermal ultrasound with real-time MR thermometry, providing closed loop feedback which ensures controlled gentle heating on a wide range of prostate sizes, and predictable outcomes within 1mm of the physician defined region. The TULSA-PRO can ablate angular sectors of the prostate with volume anywhere between 1cc to > 100cc for flexible customization of the target volume based on the patient’s disease and desired outcomes.
TULSA-PRO® is CE marked, Health Canada approved, and 510(k) cleared by the U.S. Food and Drug Administration.
If you are interested in learning more about this innovative technology, please email info@profoundmedical.com
References
- Ganzer, Roman, et al. “Which technology to select for primary focal treatment of prostate cancer?—European Section of Urotechnology (ESUT) position statement.” Prostate cancer and prostatic diseases2 (2018): 175-186.
- Van Luijtelaar, Annemarijke, et al. “Focal laser ablation as clinical treatment of prostate cancer: report from a Delphi consensus project.” World journal of urology10 (2019): 2147-2153.
- Eggener, Scott E., et al. “Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer.” The Journal of urology6 (2016): 1670-1675.
- Feller, John F., Bernadette M. Greenwood, and R. Jason Stafford. “Transrectal Laser Focal Therapy of Prostate Cancer.” Imaging and Focal Therapy of Early Prostate Cancer. Springer, Cham, (2017). 325-343.
- Walser, Eric, et al. “Focal laser ablation of prostate cancer: results in 120 patients with low-to intermediate-risk disease.” Journal of Vascular and Interventional Radiology3 (2019): 401-409.