Release of version 2.8 of the Treatment Delivery Console (TDC) software
The software team is delighted to announce the release of version 2.8 of the Treatment Delivery Console (TDC) software an integral part of the TULSA-PRO® system. Based on the feedback from our customers we have introduced a number of new features that we are excited to get into your hands.
Reports
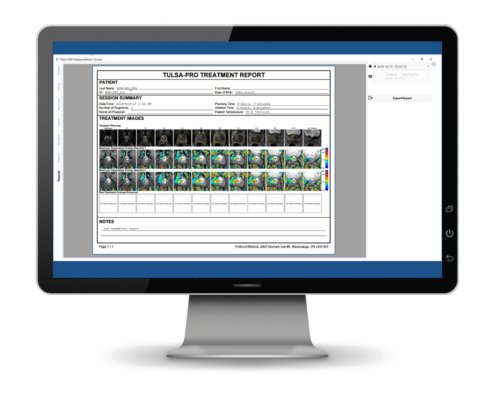
We have added a requested feature of treatment reports.
At the end of each TULSA procedure the TDC will now automatically generate a PDF report. The report contains information on the patient, session, images for the treatment and any notes or observations that the physician documented on TDC during the treatment. The images include a row each of treatment planning images, maximum thermometry images for each segment and of contrast enhanced images that are acquired at the end of the treatment. These contrast enhanced images enable the user to visualize the thermally ablated tissue in the prostate at the end of treatment.
Report
Max Temp and Thermal Dose
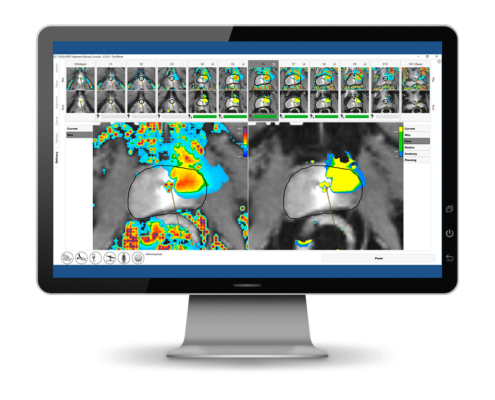
Thermal dose display mode
Another new feature is our CEM43 thermal dose display mode.
This new mode quantifies thermal exposure in terms of the number of minutes of heating at 43OC needed to obtain equivalent effects in tissue. This is a standard mechanism used to quantify the effects resulting from transient heating. The user can now easily switch between the display of maximum temperature, current temperature or the thermal dose that is overlaid on the anatomy image.
Additional changes
Other features that were added focus on improving workflow and making the TULSA-PRO software continually more user friendly.
The software team is delighted to provide you with this release and we look forward to receiving any feedback that you may have regarding the software.
Please check back here regularly for further updates from the software development team.
CAUTION: Products shown may not be approved for sale in all jurisdictions.
TULSA-PRO® has CE Marking.
Rx only device.
Contact us at info@profoundmedical.com for more information regarding the regulatory status of the device.


